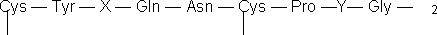
Peptide and protein hydration is a dominant factor in the stabilization of the spatial molecular structures and plays an essential role in substrate binding and in the mechanisms of peptide and protein mediated reactions. There have been several developments in the past few years which extend the NMR methodology as a powerful method for investigating the interaction of water with molecules of biological interest via 2D and 3D homonuclear NOE and ROE type dipolar cross- relaxation processes between water protons and polypeptide protons (1-3). However, to our knowledge, there is only one report in investigating the interaction of water with individual peptide protons and differential hydration of cis/trans isomers of peptides (4). In this communication we report the investigation of specific hydration phenomena of the NH protons of the neurohypophyseal peptide hormone Lys-vasopressin, cyclo (Cys-Tyr-Phe-Gln-Asn-Cys)-Pro- Lys-Gly-NH2, by the combined use of 2D-WATERGATE-NOESY and ROESY (5,6) and 1D off-resonance WATERGATE-ROESY experiments (7,8). This peptide consists of a macrocyclic hexapeptide ring, which is closed by a disulfide bond between cysteine residues at positions 1 and 6, to which a tripeptide side chain is attached via a cysteine (6) - proline peptide bond:
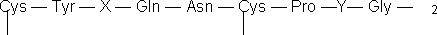
Lysine vasopressin (LVP) X=Phe Y=Lys
Arginine vasopressin (AVP) X=Phe Y=Arg
oxytocin X=Ile Y=Leu
Homonuclear 1H-1H two dimensional NOESY/ROESY experiments must be carried out in H2O solution, at low temperatures (~273K) and 2.5 < pH < 3.5 in order to minimize chemical exchange of the NH protons. Furthermore, it is necessary to suppress the water signal without using presaturation. The important feature of the pulse sequences used in these experiments is that the water signal is saturated only at the end of the mixing period during which magnetization is transferred between peptide protons and the protons of solvation molecules of water. Unfortunately, the majority of corresponding pulse schemes suffer from non-uniform excitation, baseline distortions and a low degree of water suppression. The recently proposed combination of tailored excitation with pulsed magnetic field gradients (WATERGATE) provides efficient water suppression and is well suited for investigating interactions between water and biomolecules (5,6).